
Our rich portfolio in contract pharmaceutical manufacturing contributes to better health.
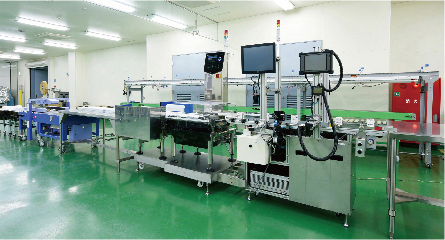
We have worked with European pharmaceutical companies since our foundation. In 1996, we began contract manufacturing of monograph drugs, generic drugs and quasi-drugs in our GMP facility. With quality, cost and speed in mind, we are dedicated to delivering stable supplies of high-quality products through our long-standing experience in production technology. From liquids, ointments, granules, and powders to other forms of products, our systematic production system is capable of full integration of manufacturing with dispensing, inspection, packaging, testing, storage and delivery for all kinds of needs, from small-scale manufacturing of multiple products to mass manufacturing.

| Factory I : | Topical disinfectants, ointments, powder dispensing |
|---|---|
| Factory II: | Granules |
| Factory III : |
Packaging [primary and secondary packaging, specialty packaging for high pharmacological activity drugs]
Tablets (sorting, filling and packaging) Secondary packaging for injectables (vials, ampules, syringes) Infusions (in bags), fixatives (PTP and strip packaging), secondary packaging for anesthetics (in glass containers) Specialty packaging for drug samples (1- to 6-tablet PTP cards and booklets) |
| Packaging Equipment | |
|---|---|
|
PTP blister line, laser carton printer/inspector (GS1-128 compatible) Table cartoner, image processor for printing and GS1-128 testing, X-ray detector for foreign materials Vial labeling system (15 to 100 mm in diameter, 35 to 150 mm in height) Vial cap labeling system Card and booklet packaging system (with laser printer and image inspector) |
|
| Pharmaceutical Storage | |
|---|---|
|
2600-pallet storage (room temperature), cold storage (2 to 8˚C), ICH stability study storage |
|
| Testing and Analysis | |
|---|---|
|
Physicochemical testing, stability testing, sterility (isolator) testing, other studies |
|













Our national and prefectural registration/certification for safe and reliable delivery:
Registered pharmaceutical manufacturer
Registered exempt narcotic manufacturer
Registered psychotropic preparations manufacturer
Quasi-drug manufacturing license
Registered general dangerous goods handler
Certified hazardous materials storage (underground alcohol storage)
Veterinary pharmaceutical manufacturing license
Registered medical device manufacturer
To ensure safety, our facility is equipped with a specialty sampling room to handle even the most sensitive products.

Copyright 2016 Kasano Kosan Co., Ltd All Rights Reserved.